SoClean 2 Support
Do you need help setting up your SoClean 2? Our Customer Care team can help you out! Call us Monday through Friday: 8:30am - 7:00pm est. at 866.501.3705. You can also send an email to info@soclean.com.
FAQ
The SoClean 2 is the fast, easy way to maintain your sleep equipment accessories. The SoClean 2 uses Activated Oxygen (ozone) in order to maintain your hose and mask daily with no water or harsh chemicals.
Wash and clean your hose and mask regularly as recommended by the manufacturer. Use mild, unscented soap and warm, drinking-quality water.
The SoClean 2 runs a 7-minute cycle plus a 2 hour rest period. This ensures proper maintenance of the equipment and the rest period allows the Activated Oxygen (ozone) to convert back into regular oxygen.
When operating your SoClean 2 per the instructions for use, if there is a noticeable scent of ozone or other unfamiliar odor or you experience headache, cough or shortness of breath, leave the room for 4 hours. Contact SoClean Customer Care before running your machine again.
If you are not satisfied with your purchase, please contact Customer Care.
The SoClean 2 is easy to use. All you need to do is place your hose and mask into the SoClean 2 Chamber, close the Lid and let the SoClean 2 do the rest. *Prior to first using your SoClean 2, we recommended that you wash, rinse and dry your hose and mask as recommended by the manufacturer to remove any facial oils or organic materials. If you would like additional information about setting up your SoClean, please contact customer care at 866-501-3705.
The SoClean 2 connects to most popular sleep equipment hoses using the Hose & Mask Adapter. If you have any questions about your particular sleep equipment, please contact customer care at 866-501-3705.
You can reset the display screen by holding down the hourglass button and manual button together until you see a smiley face icon appear on the display screen. When you see the smiley face icon, the message has been cleared and reset. Learn more about SoClean 2 Filter replacements (including how to reset your machine) here.
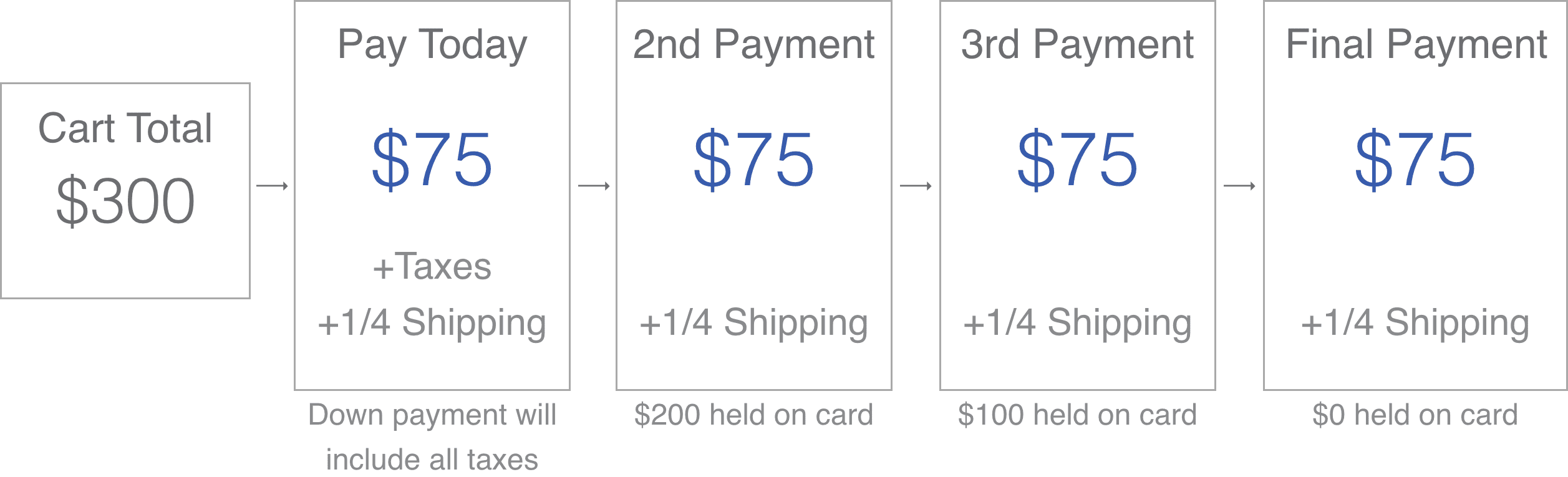
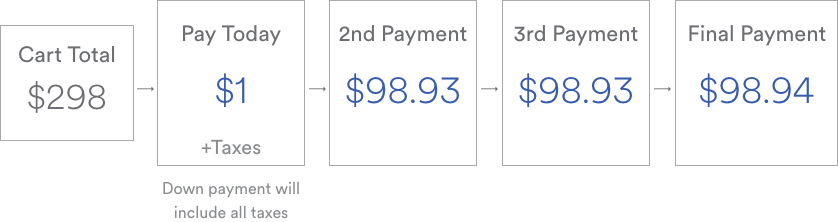
At this time the SoClean 2 is not covered by health insurance as it is considered a luxury item. SoClean 2 is eligible for FSA and HSA reimbursement, and you can use your FSA or HSA debit card on soclean.com, if all items in your cart are FSA/HSA-eligible. We also offer SoClean Easy Pay, which lets you spread your purchase over 4 easy credit card payments. More information about SoClean Easy Pay can be found here. If you have questions about either, you can contact customer care at 866-501-3705.
Philips Product Recall FAQ
Philips FAQs
If your Philips device was one of the products recalled and you have questions regarding your safety, please read this fact sheet, or visit the Philips website at: Sleep and respiratory care update | Philips..
SoClean remains confident in its product and encourages users to continue the use of their SoClean machines with the appropriate sleep equipment.
Philips users may resume use once Philips has replaced the sound abatement foam with a new material in their machines, as Philips stated in its recall notification.
Philips has conducted lab analysis of degraded foam and positively confirmed the presence of diethylene glycol, which is a degradative by-product of a chemical reaction involving polyester-based polyurethane foam and water. Philips has also said that the foam degradation was influenced by environmental conditions including high humidity and temperature.
SoClean recently filed a lawsuit against Philips alleging that the true reason for the product recall was that Philips chose a material for sound abatement – polyester-based polyurethane foam – which is “known to degrade in the presence of heat and humidity” and “off-gases harmful chemicals right out of the box.”
SoClean remains confident in its product and encourages users to continue the use of their SoClean machines with the appropriate sleep equipment.
Philips users may resume use once Philips has replaced the sound abatement foam with a new material in their machines, as Philips stated in its recall notification.
The Philips product recall was due to health risks associated with the sound abatement foam Philips chose to use in specific identified products in their Sleep and Respiratory Care portfolio. While Philips has suggested the foam degradation may be exacerbated by using an ozone cleaner, almost 90% of the products that are being recalled have no connection to an ozone cleaning device.
SoClean recently filed a lawsuit against Philips alleging that the true reason for the product recall was that Philips chose a material for sound abatement – polyester-based polyurethane foam – which is “known to degrade in the presence of heat and humidity” and “off-gases harmful chemicals right out of the box.” Many of the recalled products operate under hot and humid conditions, often with the use of a heated humidifier. Also, the off-gassing of harmful chemicals was unrelated to ozone exposure. If anything, the complaint asserts, the use of ozone cleaners would help mitigate the off-gassing of harmful chemicals by destroying them through chemical reactions.
Philips listed several potential factors that could have affected the sound abatement foam in their products, such as high heat, high humidity and the use of ozone. Philips has conducted lab analysis of degraded foam and positively confirmed the presence of diethylene glycol, which is a degradative by-product of a chemical reaction involving polyester-based polyurethane foam and water. Philips has also said that the foam degradation was influenced by environmental conditions including high humidity and temperature.
SoClean recently filed a lawsuit against Philips alleging that the true reason for the product recall was that Philips chose a material for sound abatement – polyester-based polyurethane foam – which is “known to degrade in the presence of heat and humidity” and “off-gases harmful chemicals right out of the box.” Many of the recalled products operate under hot and humid conditions, often with the use of a heated humidifier. Also, the off-gassing of harmful chemicals was unrelated to ozone exposure. If anything, the complaint asserts, the use of ozone cleaners would help mitigate the off-gassing of harmful chemicals by destroying them through chemical reactions.
SoClean remains confident in its product and encourages users to continue the use of their SoClean machines with the appropriate sleep equipment.
Philips listed several potential factors that could have affected the sound abatement foam in their products, such as high heat, high humidity and the use of ozone. Philips has conducted lab analysis of degraded foam and positively confirmed the presence of diethylene glycol, which is a degradative by-product of a chemical reaction involving polyester-based polyurethane foam and water.
Philips users may resume use once Philips has replaced the sound abatement foam with a new material in their machines, as Philips stated in its recall notification.
SoClean recently filed a lawsuit against Philips alleging that the true reason for the product recall was that Philips chose a material for sound abatement – polyester-based polyurethane foam – which is “known to degrade in the presence of heat and humidity” and “off-gases harmful chemicals right out of the box.”
Videos

Video Title:Changing the Cartridge Filter

Video Title:Soclean 2 Timer and Notifications

Video Title:Setting Up Your Soclean 2 Hose& Mask Adapter
Need a little extra help?
We are here for you! Call us at 866-501-3705 or use the contact button to send us an email.