SoClean 3 works with all popular sleep equipment brands & models.
Get the peace of mind that you're taking the best possible care of your sleep equipment and yourself, with SoClean 3.
Save $50 today + 10% More!
Promo Code SPRING24
$398 $348
FREE
SHIPPING*
30-DAY
FREE TRIAL
2-YEAR
WARRANTY
UNLIMITED
SUPPORT
U.S. LAB
TESTED
2 MILLION+
HAPPY CUSTOMERS


Rated 5.0 / 300+ Reviews
Rated 5.0 / 300+ Reviews
Does SoClean 3 work with my machine?
SoClean 3 works with all popular sleep equipment!

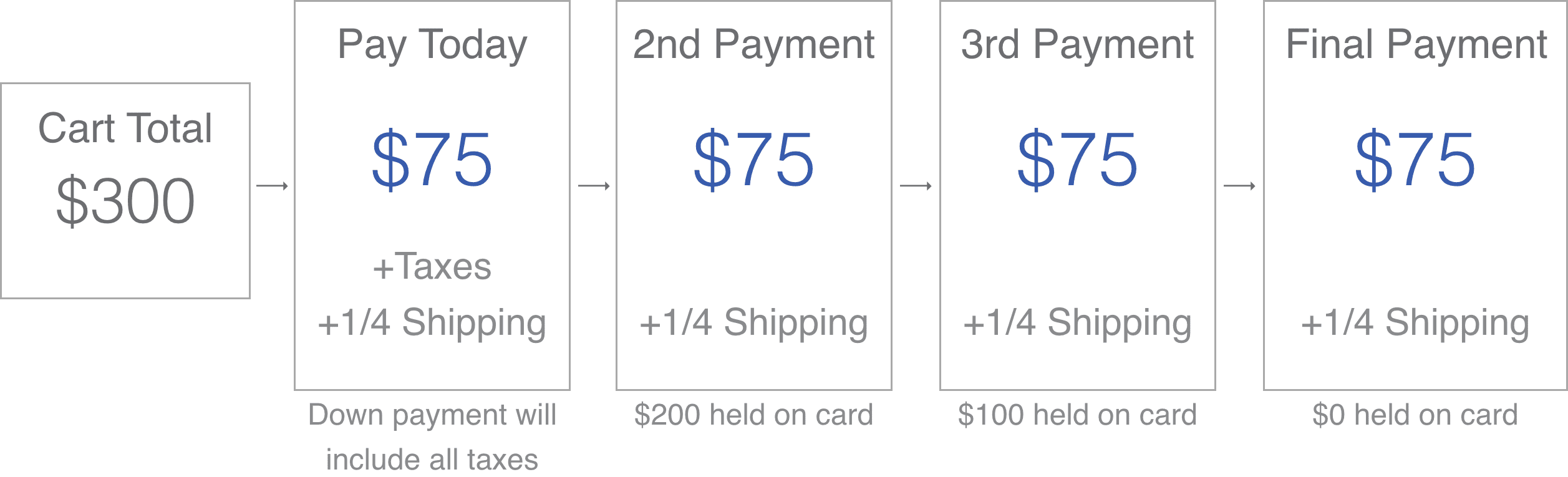
Try SoClean Today for Just $87 Down
Just 4 Monthly Installments of Only $87.
Interest-Free No Credit Check No Credit Application

Sku: SC1400-SBL
UPC: 00858242008090
Height: 9.49"
Length: 7.12"
Width: 7.36"
Weight: 3.655 lbs
Contraindications for use: Persons with underlying lung diseases, such as asthma and chronic obstructive pulmonary disease (also known as COPD, which includes emphysema and chronic bronchitis), and those with cardiovascular disease may be sensitive to ozone and should consult with their health care professional before using this product.
Replacement Filters can be purchased here.
What’s In the Box?
- 1 SoClean 3 Machine
- 1 SoClean Adapter + 2 Hose Sleeves
- 1 Filter (6-month replacement schedule)
- 1 Power Supply